

Since my sophomore year of undergraduate studies, I have been conducting research in Professor Dennis D. Cao’s organic materials lab at Macalester College. Since my debut in the lab, I have worked on two independent projects that encompass the areas of photocatalysis and supramolecular self-assembly.
The ever-worsening effects of climate change and increasing global energy demands have driven a quest for cleaner, renewable, and more sustainable energy sources. When derived from carbon-free processes, total photocatalytic splitting of water into H2 and O2 gas appeals as attractive. Using a single visible-light- responsive photocatalyst to split water into H2 and O2 requires the catalyst to have a sufficient thermodynamic potential, a narrow band gap to harvest visible photons, and stability against photocorrosion. Furthermore, the catalyst should have a large surface area as its activity is primarily a function of its surface area as to maximize chemical interactions between its redox surface and the water molecules.
Because of these stringent requirements, the number of reliable and reproducible photocatalysts that effectively use the tropospheric solar for one-step water splitting remains limited. Recently, a family of 2D visible-light activated metal-free conjugated polymers that was developed, whose photocatalytic activity was found to depend primarily on the ratio of pyrene and phenylene present in its building blocks
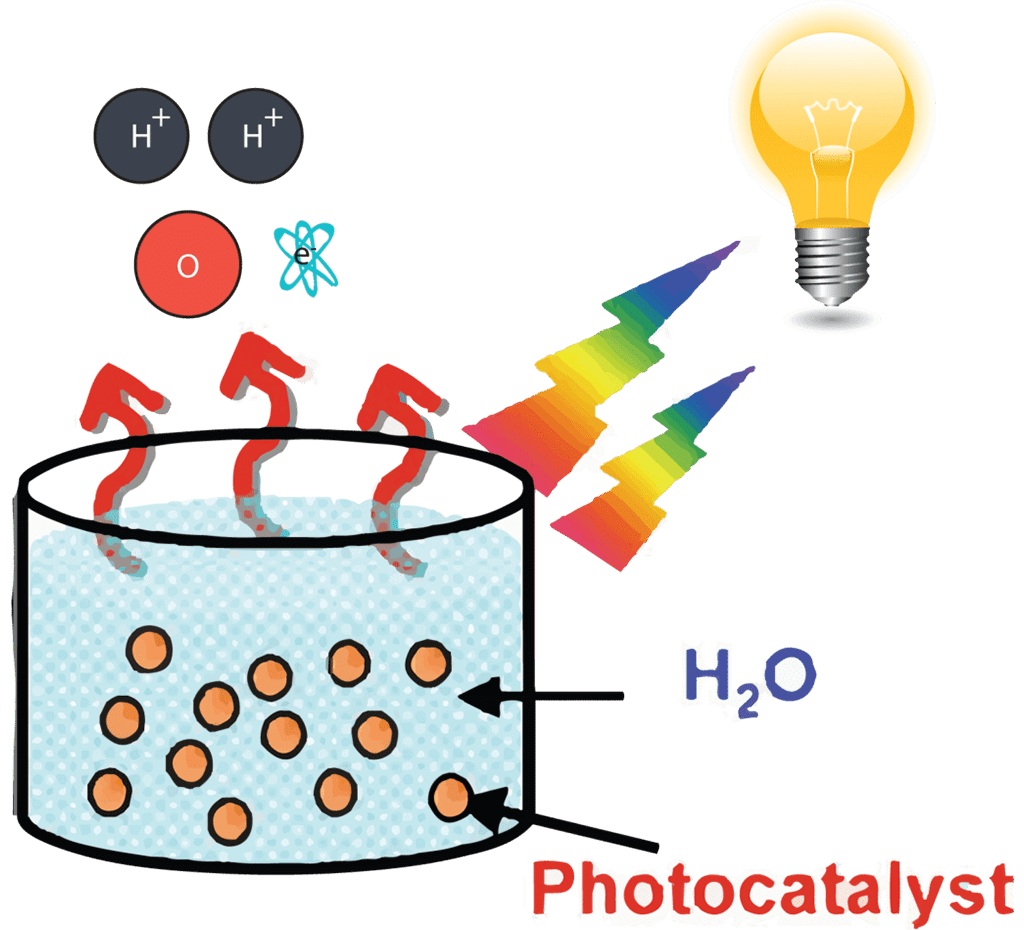
As part of my first project, I conducted an extensive study on testing metal-free visible-light active conjugated polymer photocatalysts for water splitting, and attempted to make analogous molecules of my own. One challenge I encountered was the lack of suitable instrumentation to quantify the amount of hydrogen evolved. Though we did not have a suitable mass spectrometer with a TCD detector at my institution. I instead developed a simple to use and cost-effective alternative method of quantifying hydrogen gas by using an Arduino microcontroller board, economic MQ-8 sensors and a computer program I wrote that translates the changes in the electrochemical sensor’s resistance to concentration in ppm.

Method 1: The most accurate, precise, and definitive method of detecting hydrogen is using a gas chromatograph with a thermal conductivity detector (TCD). The TCD compares the thermal conductivity of two gas flows. These are the carrier (reference) gas and the sample. Changes in the temperature of the electrically-heated wires in the detector are affected by the thermal conductivity of the gas which flows around this. The changes in this thermal conductivity are sensed as a change in electrical resistance and are measured.
Method 2: The gas sensing layer on the sensor unit is made of tin dioxide (SnO2), which has lower conductivity in clean air. The conductivity increases as the levels of hydrogen rise. The detection range of the sensor is 100-10,000 ppm of hydrogen (which seems to vary between manufacturer.