
Enamored with research and eager to continue making impactful discoveries, in the summer of 2017 I applied to and was selected to participate in a NSF international REU (iREU) program through the University of Florida, at the Université de Pierre et Marie Curie in Paris, France. This experience expanded my work in supramolecular chemistry into the realm of glycochemistry. Under the direction of Professor Matthieu Sollogoub and Dr. Olivia Bistri, I conducted research on the selective functionalization of the primary rim of alpha cyclodextrin.
Concave macrocycles have gained significant interest as potential platforms for use in an array of host-guest applications. Cyclodextrins are cavitary compounds that are highly attractive for supramolecular design since they are naturally occurring, and can be solubilized in water or organic solvents, depending on their protective groups. Due to their symmetrical cavity, however (n glucose units), selectively functionalizing them is difficult to achieve.
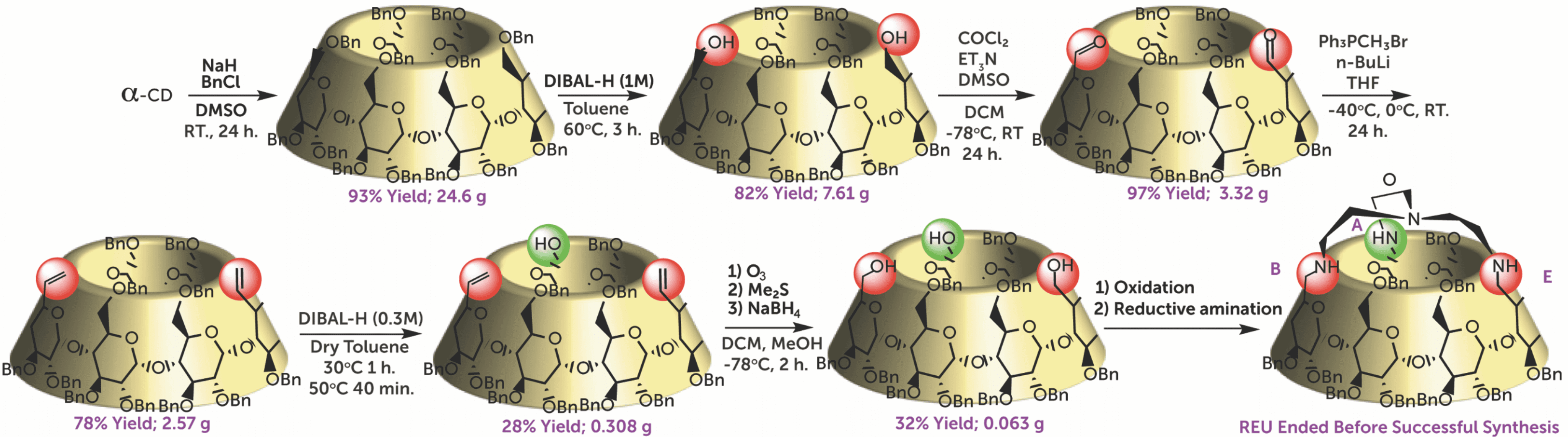
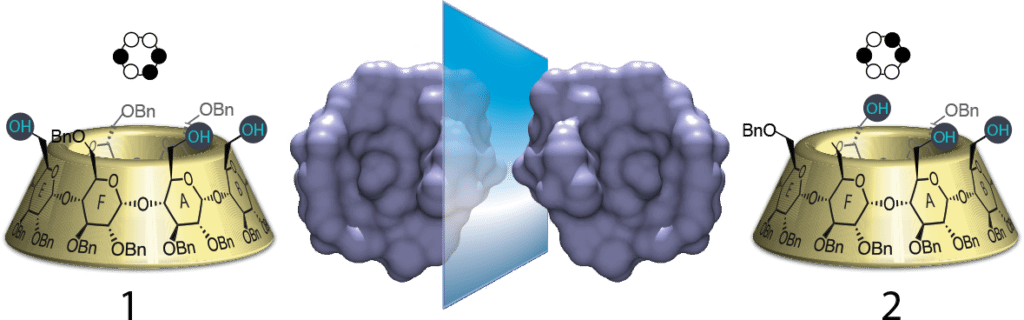
By use of selective debenzylation, we proposed a successful method by which it is possible to introduce a chiral spatial arrangement on the primary rim to then facilitate its selective modification, and efficient synthetic pathway to yield two enantiomeric spatial arrangements of primary alcohols that have potential to be used as ligands in metal-catalysis, enzyme mimics, and for improvement in the discrimination ability of chiral HPLC.

The goal of my project was to explore the effects of the substituents present on the primary rim and changes in the distortion of certain glucose units on the structure. By using selective debenzylation techniques, I was able to react hydroxyl groups at specific positions consistently, in order to access two enantiomeric triols that could serve as novel metal-catalyst ligands or offer improvement in the discrimination ability of chiral HPLC. I will also be presenting this REU research at the 255th ACS National Meeting.